Electron Diffraction + High-Performance Computing
Phase and stability analysis of pharmaceutical compounds and biomolecular polymers during drug design is a formidable challenge; for example, amorphous phase separation and drug stability under the combined effect of increased humidity and temperature impact some of the most important classes of industrial applications, involving amorphous solid dispersions (ASD) as well as crystalline organic pharmaceuticals. Often, accurate determination of the phase constituents impacts intellectual property rights.
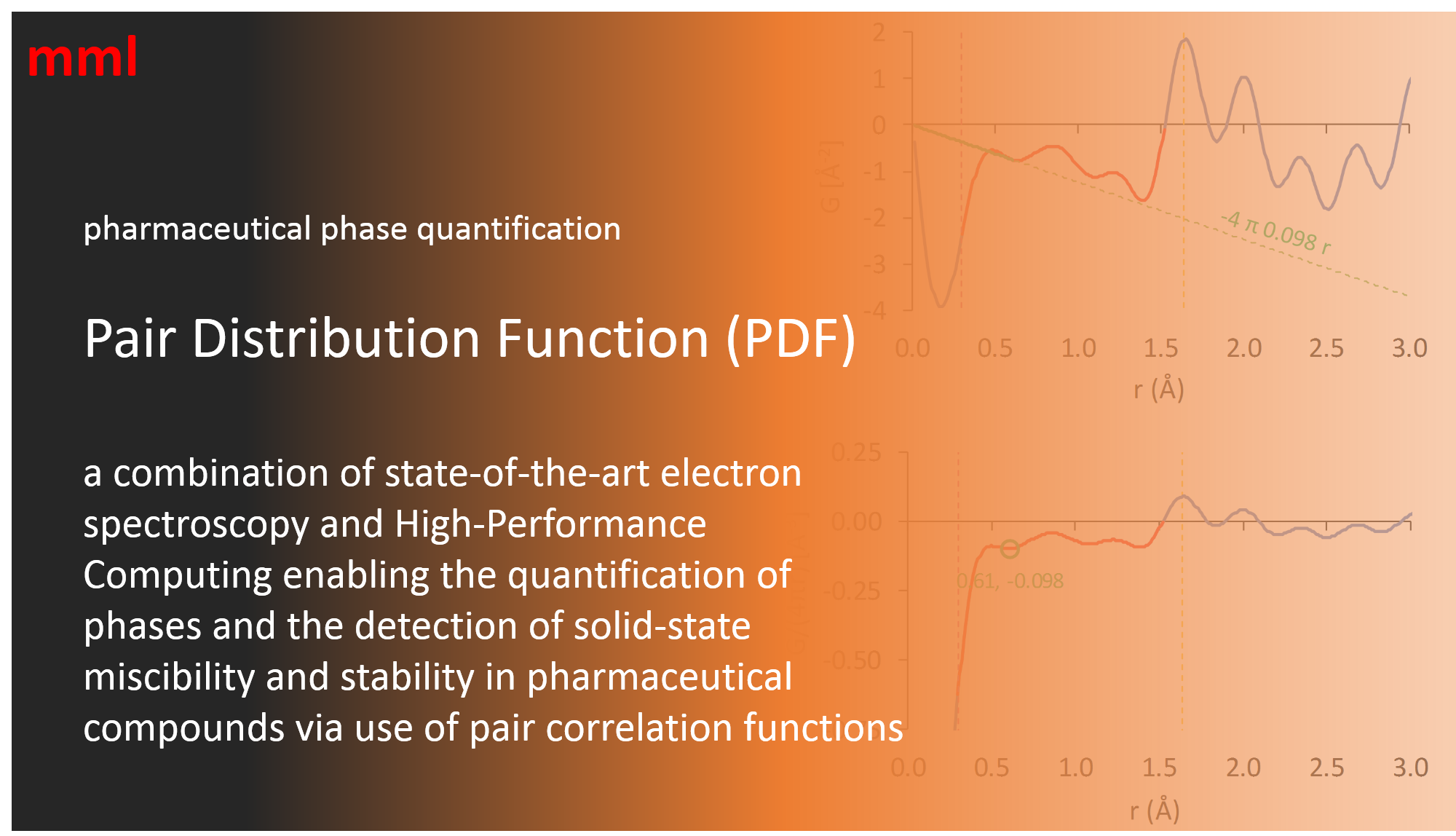
In such cases, it is feasible to definitively determine crystalline and amorphous content and associated properties via a novel combination of electron diffraction, High-Performance Computing and use of the Pair Distribution Function (PDF).
This combination of techniques has, for example, allowed us to pinpoint the solid solubility limit of the Active Pharmaceutical Ingredient (API) in a multi-component ASD without the need for further experiments.